Guide to clinical trails
Clinical trails are research studies that test new medical treatments or procedures on people. They are essential for advancing medical knowledge and developing new, more effective therapies.
A clinical trial is a research study that is done to find out if a treatment can improve people’s health. They are research studies intended to answer scientific questions to help understand whether an investigational treatment is both safe and effective for people with a particular disease or condition.
A treatment can be a drug, medical device, medical procedure, or a change in a person’s behaviour such as diet or exercise. Every modern prescription medicine we have today was studied in hundreds, and often thousands of people in clinical trials. Your decision to take part is personal. But being part of a clinical study can make a difference in the lives of other people.
People who take part in clinical trials are volunteers. They may also be called “participants” or “subjects.” When people participate in clinical trials they help contribute to medical research that finds new or better treatments for people with illnesses and diseases. The results of every clinical trial are important because they give researcher’s more information about the risks and benefits of the treatments in the trial.

Patients
Clinical trials are at the heart of research work to bring innovative medicines to people with a particular disease or condition. These studies ensure that an investigative medicine is effective and safe, and rely entirely on patients and healthy volunteers

Healthcare Professionals
Healthcare professionals are responsible for recruiting patients, administering treatments, and monitoring patient safety and outcomes. Their expertise ensures the ethical and scientific conduct of these trials.

Volunteers
Volunteers in clinical trials play a crucial role by participating in research that can lead to new treatments and medical advancements. They provide invaluable data by allowing researchers to study the safety and effectiveness of experimental drugs or medical procedures.

A Clinical Trial That’s Right For You
If you’re considering joining, you’ll be connected with a team of medical professionals who will assess if you’re a good fit for their specific study. You then go through a screening and consent process where you’ll have the opportunity to ask questions and make sure the study is also the right fit for you.
You’ll answer questions about your health and medical history (usually online or over the phone) and make an appointment to learn more.
At your appointment, you’ll spend time with the study team to review the details of the study, including possible risks and benefits, so you’ll know what to expect. If you decide to participate, you’ll give written permission for additional screenings and access to your health records.
This visit will confirm that you meet the study requirements. It includes a more detailed review of your medical history and a physical exam. It may also include additional tests related to your condition.

Who Can Participate in a Clinical Trial?
Taking part in a research study is different from regular medical care. When in a study, you’ll primarily interact with the study team—the study doctor, nurses, and potentially others who work with the doctor.
- You may have additional scheduled visits and procedures, extra laboratory tests, and/or follow a modified treatment plan.
- You can stop participating at any time—the decision to stop will not affect your regular medical care or any benefits to which you are entitled.
- Where permissible, reimbursement for study-related expenses—such as stay and travel—may be provided.
- To research a study drug for efficacy and safety, some participants are given the study drug and others are given standard of care or a placebo. Before you consent to join, you will be told how the study works.
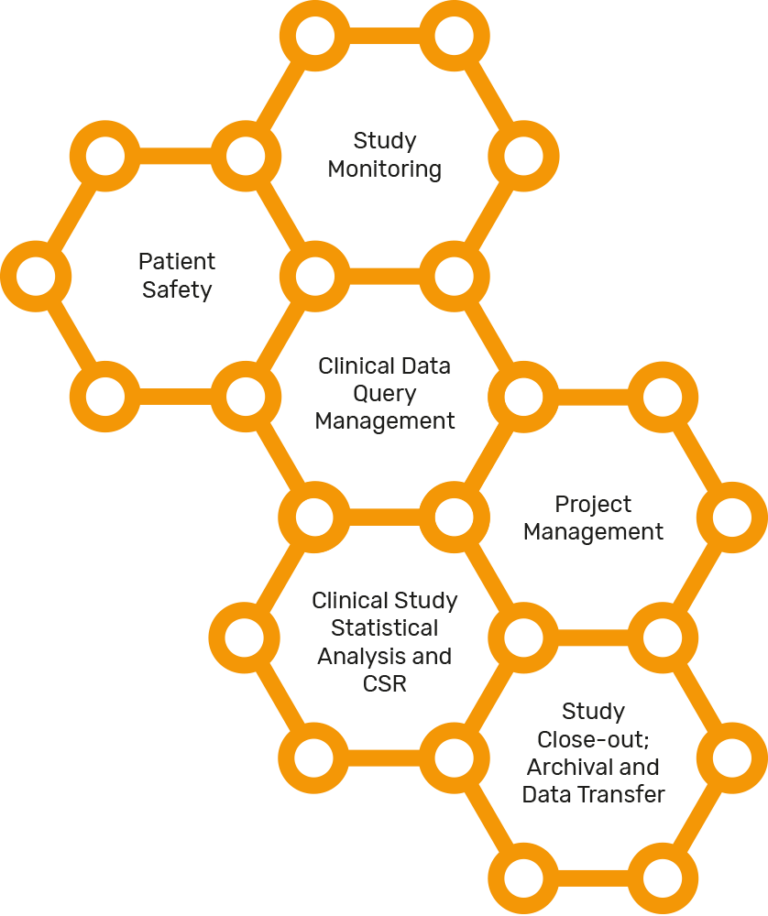
Clinical trial execution
Developing a new treatment can take a long time, sometimes even more than a decade. This is to ensure the treatment has been properly tested and is effective and safe enough for the greater public.
Before a clinical trial can start, a research plan is created. The plan details what researchers will do in the study and is designed to safeguard the well-being of the participants and to help answer specific research questions.
The research plan is also called the “protocol”. The protocol describes:
* The length of the study
* the rules about who may or may not participate in the trial
* The schedule of tests, procedures, and treatments
* The information the researchers want to collect about the treatment
The protocol is usually reviewed by an independent group of scientists and other professionals. They help make sure that the study will be safe for people with the disease or condition. Once the protocol is approved, the clinical trial can begin and participants can join.
During a clinical trial, the participants receive treatments and have tests done according to the protocol.
Some trials compare a new treatment to a standard one that is already available. Other trials compare a treatment to a placebo. A placebo looks like a treatment but does not have any medicine in it. Some trials just look at a treatment without any comparisons.
Clinical trials can take place in a variety of locations, such as hospitals, universities, doctors’ offices, or community clinics. Each location has a research team. The research team includes doctors, nurses, and other health care professionals.
The research team collects information from the participants during the trial to determine if the treatments are safe and effective. It can take months or even years to collect and review all the information.
Clinical trials are broken down into smaller steps that are called “phases”. There are four phases of trials, ranging from 1 to 4, also written as phase I – IV. Each phase has a different purpose and helps researchers answer different questions. One phase cannot conclude until it meets all of its objectives.
Earlier phases determine the safety of a treatment and any potential side effects, while later stages examine whether a new medicine is more effective than existing therapies:
* Phase I trials test an experimental drug, vaccine or device in a small group of participants (about 20-80 people) to evaluate safety, identify side effects and determine how the drug should be used or delivered. This phase can last several months.
* Phase II trials involve larger groups of participants than Phase I (about 100-300 people) and they are designed to assess whether an experimental treatment is safe and whether it works. This phase can last several years.
* Phase III trials are usually large studies (about 1000-3000 people) comparing the experimental drug or vaccine to a placebo or standard treatment, to evaluate whether the drug works and collect information to allow it to be used safely. The regulatory health authority, will consider the results of clinical trials up to and including Phase III trials when determining whether to approve a new drug or vaccine.
* Phase IV trials are performed once a drug has reached the market, to provide additional information about the best use of the drug, its risks and its benefits.
Join Rantalliz’s Clinical research registry
Learn more about the process of joining a clinical trial and find a trial near you.
Get notified about clinical trials that may be right for you — now and over time. Share some information about your health and medical areas of interest. We’ll reach out if a Rantalliz clinical trial may be a fit for you.
Sign up

Research collaborations
Our purpose at Rantalliz is to reimagine medicine to extend and improve people’s lives. To reflect this, we embrace pioneering collaborations and partner with external scientific experts to mutually benefit from ideas, knowledge, capabilities, and the complementary competencies that will enable us to innovate together.
We strongly value collaborations that will push scientific boundaries and address unmet medical need. In this regard, Rantalliz is willing to support projects with scientific merit by providing meaningful intellectual contribution, that is both acknowledged and documented, and may also provide our resources.
Read more
We define Research Collaborations as activities in which Rantalliz collaborates with one or more external organizations to advance specific, shared scientific research objectives.
Research partners may include:
Academics, Organisations, Government entities, NGOs etc.

Building success together
- Access new biological insights, innovative drug discovery technologies and novel drug modalities and drug-delivery approaches
- Support breakthrough science focused on research of mutual interest
- Gain access to drug discovery programs and drug candidates that seek to address significant unmet medical need
- Explore science outside the current internal strategy that have the potential to set future biomedical directions

